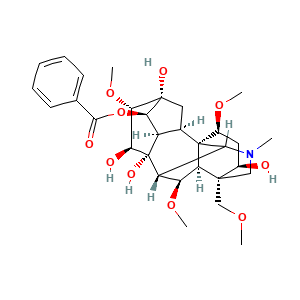
3. 结构
3.1 二维结构
3.2 三维结构
-1
-2
-3
85 91 0 1 0 0 0 0 0999 V2000
1.3173 1.3791 1.1822 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.9472 2.5977 1.3972 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.7518 -2.8478 -1.1411 O 0 0 0 0 0 0 0 0 0 0 0 0
3.0676 -0.2370 -0.7259 O 0 0 0 0 0 0 0 0 0 0 0 0
2.2103 -3.0648 -1.5110 O 0 0 0 0 0 0 0 0 0 0 0 0
0.7700 -1.2975 2.7753 O 0 0 0 0 0 0 0 0 0 0 0 0
2.8198 -2.8088 1.0315 O 0 0 0 0 0 0 0 0 0 0 0 0
-6.2482 0.1875 -0.4258 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.5410 2.9170 -1.1867 O 0 0 0 0 0 0 0 0 0 0 0 0
3.3856 1.2017 -2.4948 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.1013 -0.8128 1.6684 N 0 0 2 0 0 0 0 0 0 0 0 0
-1.9948 -0.5407 -0.5560 C 0 0 1 0 0 0 0 0 0 0 0 0
-2.4984 0.9568 -0.4582 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.5836 -0.6986 -1.2759 C 0 0 1 0 0 0 0 0 0 0 0 0
-1.8031 -0.7858 0.9602 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.8749 0.4156 1.3098 C 0 0 1 0 0 0 0 0 0 0 0 0
0.6208 0.1091 -0.7268 C 0 0 1 0 0 0 0 0 0 0 0 0
0.5851 0.1833 0.8219 C 0 0 1 0 0 0 0 0 0 0 0 0
-1.4655 1.6104 0.5071 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.9645 0.9872 0.0982 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.0232 -1.4580 -1.3013 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.0173 -2.1448 -1.3163 C 0 0 0 0 0 0 0 0 0 0 0 0
1.4478 -2.0710 -0.8449 C 0 0 2 0 0 0 0 0 0 0 0 0
1.8334 -0.6621 -1.2662 C 0 0 2 0 0 0 0 0 0 0 0 0
1.3532 -0.9788 1.5098 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.9410 0.3908 1.5397 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.8853 0.1683 -0.8536 C 0 0 1 0 0 0 0 0 0 0 0 0
1.5331 -2.2707 0.6862 C 0 0 2 0 0 0 0 0 0 0 0 0
-4.4889 -1.2845 -0.9502 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.5517 2.4212 0.1315 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9661 -1.2174 3.0644 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.8949 3.4122 1.9040 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.1939 -3.5942 -2.2648 C 0 0 0 0 0 0 0 0 0 0 0 0
3.7511 0.7048 -1.4408 C 0 0 0 0 0 0 0 0 0 0 0 0
2.7614 -3.5119 2.2587 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.0656 4.2407 -1.2643 C 0 0 0 0 0 0 0 0 0 0 0 0
5.0262 1.0638 -0.7702 C 0 0 0 0 0 0 0 0 0 0 0 0
5.8735 2.0116 -1.3441 C 0 0 0 0 0 0 0 0 0 0 0 0
5.3770 0.4527 0.4336 C 0 0 0 0 0 0 0 0 0 0 0 0
7.0717 2.3486 -0.7141 C 0 0 0 0 0 0 0 0 0 0 0 0
6.5751 0.7896 1.0636 C 0 0 0 0 0 0 0 0 0 0 0 0
7.4223 1.7375 0.4897 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.4531 1.4320 -1.4455 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.7262 -0.3805 -2.3195 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3094 -1.7455 1.1335 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.8776 0.6257 2.3844 H 0 0 0 0 0 0 0 0 0 0 0 0
0.6558 1.1124 -1.1664 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.7374 2.1134 -0.1336 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.9234 -1.1916 -2.3646 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.0811 -2.5279 -2.3431 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.5557 -2.8591 -0.6932 H 0 0 0 0 0 0 0 0 0 0 0 0
1.8676 -0.6194 -2.3644 H 0 0 0 0 0 0 0 0 0 0 0 0
2.3588 -0.6166 1.7657 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.6117 1.1487 2.2596 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.9655 0.1381 1.8418 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.8596 0.6005 -1.8620 H 0 0 0 0 0 0 0 0 0 0 0 0
0.7792 -3.0138 0.9771 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.7522 -1.8344 -0.0375 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.1226 -1.7573 -1.7119 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.5832 2.4001 0.5060 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.0093 3.1026 0.7870 H 0 0 0 0 0 0 0 0 0 0 0 0
1.3608 1.4194 2.1528 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.4328 -2.1712 3.1431 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.9526 -1.3757 3.5141 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.4335 -0.4741 3.6666 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1691 -2.8924 -2.4669 H 0 0 0 0 0 0 0 0 0 0 0 0
0.8366 -0.5065 3.3367 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.7772 -0.2745 -1.0985 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2803 3.8287 1.1000 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3537 4.2469 2.4421 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2829 2.8598 2.6199 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8569 -4.6264 -2.1309 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.7512 -3.2171 -3.1922 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.2837 -3.6132 -2.3397 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7127 -4.0381 2.3846 H 0 0 0 0 0 0 0 0 0 0 0 0
1.9655 -4.2635 2.2626 H 0 0 0 0 0 0 0 0 0 0 0 0
2.6567 -2.8321 3.1073 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.1043 4.2604 -0.9211 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.4563 4.9279 -0.6693 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.0351 4.5602 -2.3093 H 0 0 0 0 0 0 0 0 0 0 0 0
5.6186 2.4990 -2.2813 H 0 0 0 0 0 0 0 0 0 0 0 0
4.7485 -0.2912 0.9141 H 0 0 0 0 0 0 0 0 0 0 0 0
7.7316 3.0865 -1.1608 H 0 0 0 0 0 0 0 0 0 0 0 0
6.8489 0.3138 2.0007 H 0 0 0 0 0 0 0 0 0 0 0 0
8.3552 1.9997 0.9802 H 0 0 0 0 0 0 0 0 0 0 0 0
1 18 1 0 0 0 0
1 62 1 0 0 0 0
2 19 1 0 0 0 0
2 32 1 0 0 0 0
3 21 1 0 0 0 0
3 33 1 0 0 0 0
4 24 1 0 0 0 0
4 34 1 0 0 0 0
5 23 1 0 0 0 0
5 66 1 0 0 0 0
6 25 1 0 0 0 0
6 67 1 0 0 0 0
7 28 1 0 0 0 0
7 35 1 0 0 0 0
8 27 1 0 0 0 0
8 68 1 0 0 0 0
9 30 1 0 0 0 0
9 36 1 0 0 0 0
10 34 2 0 0 0 0
11 15 1 0 0 0 0
11 26 1 0 0 0 0
11 31 1 0 0 0 0
12 13 1 0 0 0 0
12 14 1 0 0 0 0
12 15 1 0 0 0 0
12 21 1 0 0 0 0
13 19 1 0 0 0 0
13 20 1 0 0 0 0
13 43 1 0 0 0 0
14 17 1 0 0 0 0
14 22 1 0 0 0 0
14 44 1 0 0 0 0
15 16 1 0 0 0 0
15 45 1 0 0 0 0
16 18 1 0 0 0 0
16 19 1 0 0 0 0
16 46 1 0 0 0 0
17 18 1 0 0 0 0
17 24 1 0 0 0 0
17 47 1 0 0 0 0
18 25 1 0 0 0 0
19 48 1 0 0 0 0
20 26 1 0 0 0 0
20 27 1 0 0 0 0
20 30 1 0 0 0 0
21 29 1 0 0 0 0
21 49 1 0 0 0 0
22 23 1 0 0 0 0
22 50 1 0 0 0 0
22 51 1 0 0 0 0
23 24 1 0 0 0 0
23 28 1 0 0 0 0
24 52 1 0 0 0 0
25 28 1 0 0 0 0
25 53 1 0 0 0 0
26 54 1 0 0 0 0
26 55 1 0 0 0 0
27 29 1 0 0 0 0
27 56 1 0 0 0 0
28 57 1 0 0 0 0
29 58 1 0 0 0 0
29 59 1 0 0 0 0
30 60 1 0 0 0 0
30 61 1 0 0 0 0
31 63 1 0 0 0 0
31 64 1 0 0 0 0
31 65 1 0 0 0 0
32 69 1 0 0 0 0
32 70 1 0 0 0 0
32 71 1 0 0 0 0
33 72 1 0 0 0 0
33 73 1 0 0 0 0
33 74 1 0 0 0 0
34 37 1 0 0 0 0
35 75 1 0 0 0 0
35 76 1 0 0 0 0
35 77 1 0 0 0 0
36 78 1 0 0 0 0
36 79 1 0 0 0 0
36 80 1 0 0 0 0
37 38 2 0 0 0 0
37 39 1 0 0 0 0
38 40 1 0 0 0 0
38 81 1 0 0 0 0
39 41 2 0 0 0 0
39 82 1 0 0 0 0
40 42 2 0 0 0 0
40 83 1 0 0 0 0
41 42 1 0 0 0 0
41 84 1 0 0 0 0
42 85 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
[(1S,2R,3R,4R,5R,6S,7S,8R,9R,13R,14R,16S,17S,18R)-5,7,8,14-tetrahydroxy-6,16,18-trimethoxy-13-(methoxymethyl)-11-methyl-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-4-yl] benzoate
4.2 InChl
InChI=1S/C31H43NO10/c1-32-13-28(14-38-2)17(33)11-18(39-3)30-16-12-29(36)25(42-27(35)15-9-7-6-8-10-15)19(16)31(37,24(34)26(29)41-5)20(23(30)32)21(40-4)22(28)30/h6-10,16-26,33-34,36-37H,11-14H2,1-5H3/t16-,17-,18+,19-,20+,21+,22-,23?,24+,25-,26+,28+,29-,30+,31-/m1/s1
4.3 InChlKey
PULWZCUZNRVAHT-IJNXHYLPSA-N
4.4 Canonical SMILES
CN1CC2(C(CC(C34C2C(C(C31)C5(C6C4CC(C6OC(=O)C7=CC=CC=C7)(C(C5O)OC)O)O)OC)OC)O)COC
4.5 lsomeric SMILES
CN1C[C@@]2([C@@H](C[C@@H]([C@@]34[C@@H]2[C@H]([C@@H](C31)[C@@]5([C@@H]6[C@H]4C[C@@]([C@@H]6OC(=O)C7=CC=CC=C7)([C@H]([C@@H]5O)OC)O)O)OC)OC)O)COC
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病